Protein Engineering
Proteins are the workhorses of life that carry out nearly every biological task inside living organisms, from breaking down food to fighting infections. But what if we could redesign proteins to make them stronger, faster, or capable of doing things nature never intended? That is exactly what protein engineering is all about.
Introduction.
Protein engineering is a field related to biotechnology in which designing or building new proteins and modifying pre-existing proteins, and also increasing its stability, function, and activity. Proteins are made up of amino acids, and their fold is made up of a three-dimensional structure, and its structure is found by their its function. By changing the sequence of amino acids, scientists can create proteins with improved or new functions that do not exist naturally. This field combines molecular biology, genetics, chemistry, and computational biology.
How Protein Engineering Works.
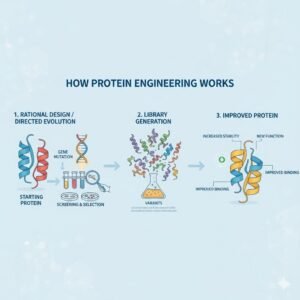
Protein engineering is a powerful scientific method that modifies or designs proteins to perform specific functions and possibly improve the properties associated with the natural macromolecule. The reason this discipline is so powerful is that proteins are the molecular machines of life responsible for catalyzing reactions, transporting molecules, and giving structural stability. Hence, engineering proteins allows scientists to create improved forms of enzymes, medicines, and materials applied in a variety of industries. The act of protein engineering encompasses biology, chemistry, and computation; specifically, it alters the amino acid sequence of the target protein to achieve a level of performance not found in the version of protein derived from nature.
Protein engineering is generally achieved through three main approaches:
1. Rotation design
- Researchers use structural knowledge of proteins (with techniques such as X-ray crystallography, NMR, or AI-assisted methods such as AlphaFold) to speculate how mutations can influence function.
Site-directed mutagenesis is then utilized to make changes.
Here’s an example: increase enzyme stability in detergents.
2. Directed evolution
- Motivated by natural selection. Scientists assemble libraries of variants and screen them to find variants with traits no biologist could design de novo. After several rounds of selection, they create proteins with very good efficiency. For instance, Frances Arnold’s Nobel Prize-winning work illustrates how to evolve industrial enzymes.
3. Hybrid approaches
- Hybrid strategies in protein engineering harness the benefits of both rational design and directed evolution to formulate improved approaches to design and improve proteins. Rational design uses sophisticated and specific insights into a protein’s structure and function to change a protein in a targeted fashion. Directed evolution uses genetic random mutagenesis to allow for random mutations and selection of more enhanced versions of proteins. Hybrid strategies have benefits and drawbacks. Rational design can be precise, but requirements for a complete understanding of proteins can be hampered by practical limitations. Directed evolution appears to yield huge improvement potential; however, it is constrained by a vast screening process. Hybrid strategies allow scientists to make more precise protein modifications in a more predictable fashion.
Applications of Protein Engineering
1. Medicine
- In medicine field protein engineering is important because it plays an important role in synthesizing proteins, enzymes, and vaccines. For example, today we synthesized insulin and human growth hormone through DNA technology, because they provide safe and secure treatment against diabetes and growth disorders. Many cancer antibodies created through this technique, like monoclonal antibodies. and also use other autoimmune diseases and viral infections. This technique is also used in the pharmaceutical field for preparing many drugs.
2. Industry
- In industrial biotechnology, protein engineering further provides engineered enzymes to function better in extreme environmental conditions, particularly temperature and pH. These engineered and naturally occurring enzymes have been used in producing detergents, textiles, paper, and biofuels, all of which contribute to efficiency while being more environmentally sustainable. For example, engineered lipases and proteases have provided improved cleaning power for detergents, while thermophilic enzymes increase heat-tolerant cycles for bioethanol production.
3. Agriculture
- In agricultural fields, protein engineering plays an important role in modern agriculture. A scientist modifies the seed protein to enhance the crop’s productivity and increase plant growth, and also protects against diseases and environmental stress like drought and extreme temperature. This technique provides more fields to global needs. This technique is useful for insect attacks on the fields, as they reduces the probability of attacks on the fields.
Techniques Used in Protein Engineering.
- Site-directed mutagenesis – specific alterations of amino acids.
- Random mutagenesis – generating a multitude of variants.
- DNA shuffling – shuffling DNA fragments.
- Phage display – presenting proteins for selection.
- Computational design & AI – predicting structure & function.
The Future of Protein Engineering.

Advances in technology, particularly artificial intelligence and machine learning, are paving the way for the future of the field. The use of tools like AlphaFold has changed the way researchers think about protein structure prediction, becoming more efficient and effective in the rational design of proteins. Some future directions are:
- Engineering synthetic proteins to perform functions not possible in nature.
- Create eco-friendly enzymes and replace to harmful chemical processes.
- Developing patient-specific therapeutic proteins in the realm of personalized medicine.
- Creating the next generation of CRISPR-based systems to be able to edit genomes more accurately.
Approaches in Protein Engineering.
1. Rational Design
- Informed by a comprehensive understanding of protein structure, function, and folding.
- Based on predictions of how any change in the amino acid sequence will change function.
- Example approaches: site-directed mutagenesis, computational modeling.
Advantages:
- Specific and focused.
- Fewer experiments.
Disadvantages:
- They require structural data of high quality (X-ray crystallography, NMR, Cryo-EM).
- More complex since protein folding may be unpredictable.
2. Directed Evolution
- A process of natural evolution occurring in the laboratory.
- Process:
- Create a library of protein variants (random mutagenesis, DNA shuffling).
- Screen for/select variants with improved function.
- Repeat for several rounds to accumulate beneficial mutations.
Advantages:
- No need to understand intricate structures.
- Possible discovery of mutant beneficial mutations..
Disadvantages:
- Labor-intensive and can require high-throughput screening.
- Example: Frances Arnold (Nobel Laureate 2018) was the first to perform directed evolution to create an enzyme for the industrial applications.
3. Hybrid Approaches
- Combining rational design (for specific improvements) with directed evolution (for a wide exploration).
- Accelerates the discovery of efficient and stable proteins.
Conclusion.
Protein engineering is changing the future of science and technology. Whether that ends up being in the context of life-saving drugs or sustainable industries, engineered proteins are at the forefront of innovation. The future looks limitless as scientists are combining artificial intelligence with genetic engineering and molecular biology.
Essentially, protein engineering allows us to redesign the building blocks for life, ultimately for benefit for health, the environment, and society.