DNA is the molecule that carries the genetic instructions essential for the development, functioning, growth, and reproduction of all living organisms and many viruses.DNA is made up of nucleotides. Each nucleotide consists of: A phosphate group, A deoxyribose sugar (5-carbon sugar), and A nitrogenous base (Adenine, Thymine, Cytosine, or Guanine). DNA has a double helix structure, discovered by James Watson and Francis Crick in 1953. The two strands are held together by hydrogen bonds between the nitrogenous bases: Adenine (A) pairs with Thymine (T), Cytosine (C) pairs with Guanine (G).
Component of DNA.
DNA (Deoxyribonucleic Acid) is made up of nucleotides, and each nucleotide has three components:
- Phosphate group (PO₄³⁻)
- Deoxyribose sugar (a 5-carbon sugar)
- Nitrogenous base (Adenine, Thymine, Cytosine, or Guanine)
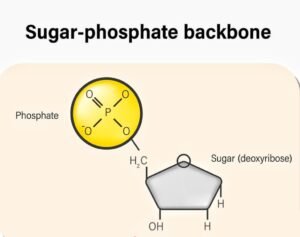
1. Phosphate Group( PO ₄ ³ ⁻).
- There is a negatively charged molecule.
- They form part of the backbone of the DNA strand.
- They link with the sugar of the next nucleotide to form a sugar-phosphate backbone.
Structure:
- One phosphorus (P) atom is at the center.
- It is bonded to four oxygen (O) atoms:
- One of these bonds is a double bond (P=O).
- Two oxygen atoms carry a negative charge (O⁻).
- One oxygen is bonded to the deoxyribose sugar via an ester bond.
Function:
1. Phosphate Group (PO₄³⁻)
- The phosphate group links adjacent nucleotides via phosphodiester bonds:
- They connect to the 3′ hydroxyl group of one sugar to the 5′ phosphate group of the next sugar.
- Creates the strong, stable sugar-phosphate backbone of the DNA strand.
2. Gives DNA Directionality
- Phosphate groups help define the 5′ to 3′ direction of DNA strands:
- One end has a free 5′ phosphate group
- The other end has a free 3′ hydroxyl group
3. Provides Negative Charge
- Each phosphate group carries a negative charge:
- This makes the entire DNA molecule negatively charged
- Influences how DNA interacts with proteins (like histones) and how it behaves in electrophoresis
4. Contributes to DNA Stability
- The sugar phosphate backbone is strong and resistant to cleavage.
- Protects the genetic code stored in the nitrogenous bases from degradation.
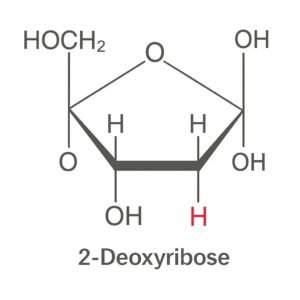
2. Deoxyribose sugar (a 5-carbon sugar).
- A 5-carbon sugar (pentose) is specific to DNA.
- Called 2-deoxyribose because it lacks an oxygen atom at the 2′ carbon.
- Forms the central part of the nucleotide and connects:
- 1′ carbon → to a nitrogenous base
- 5′ carbon → to a phosphate group
- 3′ carbon → to the next nucleotide via phosphodiester bond
Structure:
- 5 carbons numbered 1′ to 5′.
- They form a five-membered ring (furanose form) with an oxygen atom.
- 1′ carbon bonds to a nitrogenous base (A, T, C, or G).
- 3′ carbon has a hydroxyl group (-OH) that forms a bond with the phosphate group of the next nucleotide.
- 5′ carbon is attached to the phosphate group.
- The 2′ carbon has only a hydrogen (-H), no hydroxyl (-OH), which is what makes it “deoxy”.
Function:
- Forms the sugar backbone of DNA.
- The absence of the 2′ hydroxyl group makes DNA more chemically stable than RNA.
- They provide structural stability and explain the directionality 5′ to 3′ prime of DNA strands.
3. Nitrogenous base.
There are four types of nitrogenous bases in DNA, divided into two groups:
1. Purines (2-ring structures)
- Adenine (A)
- Guanine (G)
2. Pyrimidines (1-ring structures)
- Cytosine (C)
- Thymine (T)
1. Adenine (A).
- Adenine is one of the four main nitrogenous bases found in DNA.
- It is a purine base, which means it has a double-ring structure.
- It pairs specifically with Thymine (T) in DNA.
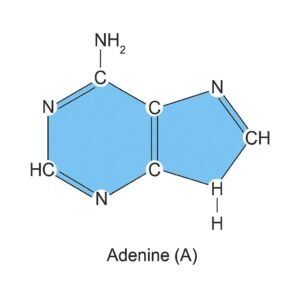
Structure:
- They contain two fused rings: a six-membered and a five-membered nitrogen-containing ring.
- Molecular formula: C₅H₅N₅
- Key atoms: carbon (C), hydrogen (H), and nitrogen (N)
Function:
- Genetic Information Storage.
- Base Pairing and DNA Stability.
- Energy Transfer (Outside DNA).
- Cell Signaling.
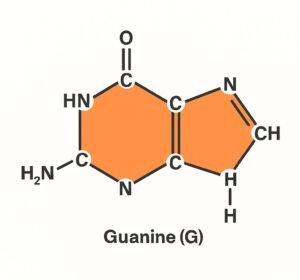
2. Guanine (G).
- It is one of the four main nitrogenous bases in DNA and RNA.
- There are the purines, meaning it has a double-ring structure (a fused six-membered and five-membered ring).
- It pairs specifically with Cytosine (C) in DNA and RNA.
Structure:
- Molecular formula: C₅H₅N₅O
- Contains nitrogen and oxygen atoms that allow hydrogen bonding.
- Has a keto group (=O) and an amino group (–NH₂) involved in base pairing.
Function:
- Genetic Information Storage.
- Complementary Base Pairing.
- DNA Stability: G≡C pairing (3 hydrogen bonds) provides extra stability
- Role in RNA & Energy.
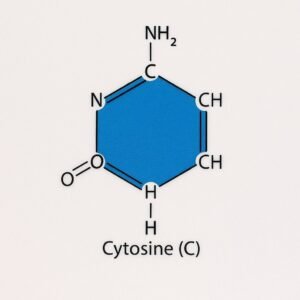
3. Cytosine (C).
- Cytosine is one of the four nitrogenous bases found in both DNA and RNA.
- It is a pyrimidine, meaning it has a single-ring structure.
- In DNA, cytosine pairs specifically with guanine (G).
Structure:
- Molecular formula: C₄H₅N₃O. It contains:
- One six-membered ring with carbon and nitrogen atoms
- An amino group (–NH₂)
- A keto group (=O)
Function:
- Genetic Information Storage
- Base Pairing: Pairs with guanine to ensure DNA stability and fidelity.
- DNA Replication & Transcription.
- Methylation Site
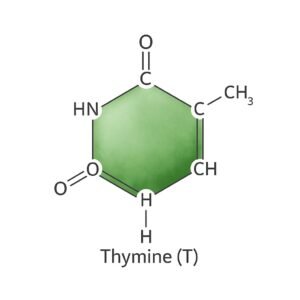
4. Thymine (T).
- There are four main nitrogenous bases found in DNA.
- It is a pyrimidine base, which means it has a single-ring structure.
- Thymine pairs specifically with Adenine (A) in DNA.
Structure:
- They have a single six-membered ring with nitrogen and carbon atoms.
- Molecular formula: C₅H₆N₂O₂
- Contains oxygen atoms (keto groups) that participate in hydrogen bonding.
Function:
- Genetic Information Storage
- Base Pairing and DNA Stability
- Differentiation from RNA, Thymine is exclusive to DNA (not found in RNA).




